WELCOME TO THE PHARMACY WORLD
Geometric isomerism can arise when there is lack of free rotation around a bond, often a C = C bond.
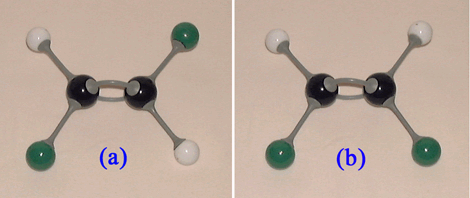
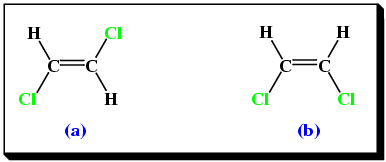
Consider 1,2-dichloroethene. There are two geometric isomers (Table 3).
|
They exist because rotation about the C = C bond is very difficult.

In Topic 2, the C = C
bond was described as consisting of a
![]() bond and a
bond and a ![]() bond (Figure
2).
bond (Figure
2).
|
Rotation is restricted because this would involve breaking the
![]() bond. The two geometric isomers are not superimposable.Geometric isomers
caused by restricted rotation around a bond are distinguished by
bond. The two geometric isomers are not superimposable.Geometric isomers
caused by restricted rotation around a bond are distinguished by
using the terms - 'cis' and 'trans':
- cis - both groups are on the same side of the double bond
- trans - the groups are on opposite sides of the double bond ('trans' means across)
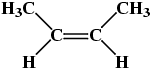
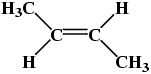
Molecule(a) in Table 3 is trans-1,2-dichloroethene, while molecule(b) in Table 3 is cis-1,2-dichloroethene. A similar situation occurs with alkenes containing 4 or more carbon atoms, e.g.
|
|
|
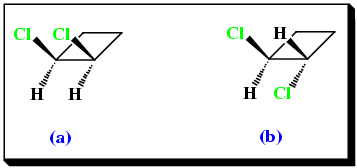
Geometric isomerism can also arise in disubstituted cycloalkanes, e.g.
|
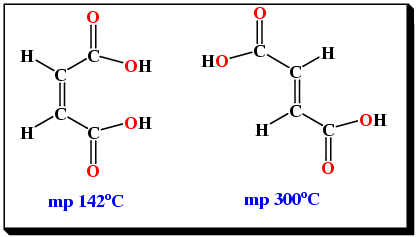
Sometimes geometric isomers can show differences in chemical properties although this is much less common than for structural isomers. A simple example involves the geometric isomers of butenedioic acid (Figure 6).
|
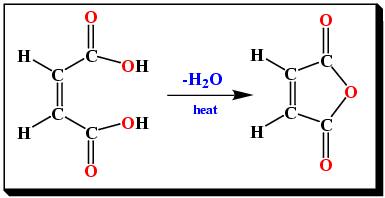
Cis-butenedioic acid readily eliminates water on heating. The product is known as a cyclic anhydride (Figure 7).
|
On the other hand, trans-butenedioic acid cannot eliminate water under the same conditions since the two carboxyl groups are on opposite sides of the double bond (Figure 6).