|
|
|
Geometric Isomerism results most commonly from
Carbon-Carbon double bonds. The important property which introduces the
feature is the inability of the Carbon atoms to rotate relative to one another
about the double bond. This is due specifically to the Pi bond but I won't
discuss this part of the subject further on this site.
The lack of rotation means the same groups can be attached in different ways to
achieve diastereomers. The molecules have identical connectivity so can't be
described as structural isomers.
![]()
![]()
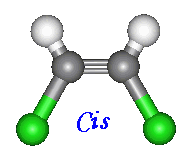
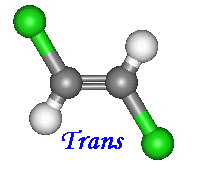
There are two ways to name compounds of this kind. When
two of the four groups attached to the carbons involved are hydrogen, the cis
and trans notation can be used. When the other two constituents are on
the same side of the molecules name is given the prefix 'cis'. Otherwise
if the constituents are on opposite sides the prefix is 'trans'.
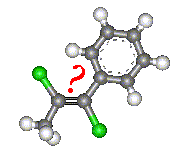 With molecules such as that shown in the image, it is not
possible to assign a cis or trans prefix which unambiguously describes the
molecule. Therefore there is a second a second convention which can be used to
name any geometric isomer. This gives us the E and Z isomers.
With molecules such as that shown in the image, it is not
possible to assign a cis or trans prefix which unambiguously describes the
molecule. Therefore there is a second a second convention which can be used to
name any geometric isomer. This gives us the E and Z isomers.
Each substituent of the double bond can
be given a priority. By looking at the
highest priority groups on each of the carbons we say if both are on the same
side (as with cis) this is the 'Z' isomer. If they are on opposite sides
we say it is the 'E' isomer .
Priority is determined by the atomic number of the atoms in the groups. Atoms of
higher atomic number are given higher priority. If the first atoms are the same,
you move to the next atom, and keep doing so until a difference can be found.
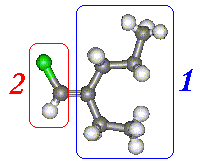 On Carbon 1 we have Ethyl and a Propyl
groups. Moving away from the Carbon in the double bond the first difference is
at the second carbon atom. The group at the top has a Carbon, whereas the group
at the bottom has a hydrogen. The Carbon is clearly of higher atomic number and,
therefore, priority.
On Carbon 1 we have Ethyl and a Propyl
groups. Moving away from the Carbon in the double bond the first difference is
at the second carbon atom. The group at the top has a Carbon, whereas the group
at the bottom has a hydrogen. The Carbon is clearly of higher atomic number and,
therefore, priority.
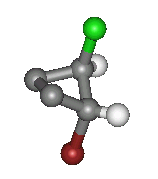
On Carbon 2 there is a Chlorine on the top and a Hydrogen at the bottom. The Chlorine is the higher priority group.
As both high priority groups are on the same side this is the 'Z' isomer.
There are also examples of this sort of isomerism where
no double bond is present. One example of this is disubstituted cycloalkanes.
There is no double bond but the rigidity introduced by the ring introduces
geometric isomerism.
Here the Bromine is pointing down from the ring and the Chlorine is pointing up.
This is another type of geometric isomerism, the molecule shown is the 'trans'
form. Hydrogens have been left off this image to make it clearer to see the
sites of interest.