|
|
|
WELCOME TO THE PHARMACY WORLD
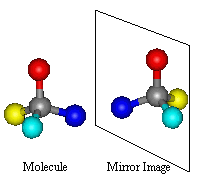 As this is the type of isomerism which can be found in
thalidomide, it is of special interest to us here. With optical isomerism, there
is no difference in connectivity and no double bonds. The isomerism is to do
with the arrangement of the atoms in space. It arises through the presence of a
Chiral Centre. Optical isomers are Non Superimposable Mirror Images
of each other; a set of optical isomers are called enantiomers.
As this is the type of isomerism which can be found in
thalidomide, it is of special interest to us here. With optical isomerism, there
is no difference in connectivity and no double bonds. The isomerism is to do
with the arrangement of the atoms in space. It arises through the presence of a
Chiral Centre. Optical isomers are Non Superimposable Mirror Images
of each other; a set of optical isomers are called enantiomers.
Enantiomers can not be interconverted without breaking bonds. They have mostly identical physical properties and can often only be told apart by what we call Optical Activity. This is the observation that in a pair of enantiomers one will rotate plane polarised light clockwise, and the other an equal amount anticlockwise.
The Chiral Centre is an atom connected to Four Different Subsituent Groups. If there is one chiral atom in a molecule there will be two enantiomeric forms. A molecule with two chiral centres however need not be Chiral!
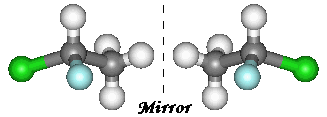 Organic compounds give endless examples of chirality.
Here, I have pictured 1,1-chlorofluoroethane (an example of a CFC). Carbon1
has a hydrogen, a chlorine, a fluorine and a methyl group attached to it making
it a chiral centre. It therefore exists in these two enantiomeric forms. The two
molecules cannot be superimposed on one another.
Organic compounds give endless examples of chirality.
Here, I have pictured 1,1-chlorofluoroethane (an example of a CFC). Carbon1
has a hydrogen, a chlorine, a fluorine and a methyl group attached to it making
it a chiral centre. It therefore exists in these two enantiomeric forms. The two
molecules cannot be superimposed on one another.
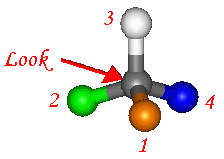 We need an unambiguous method for
assigning absolute configuration to a chiral centre. There are a few
simple steps which allow us to assign either 'R' or 'S' configuration to chiral
atoms. These configurations are then included in the name to enable
differentiation.
We need an unambiguous method for
assigning absolute configuration to a chiral centre. There are a few
simple steps which allow us to assign either 'R' or 'S' configuration to chiral
atoms. These configurations are then included in the name to enable
differentiation.
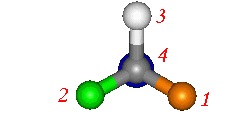
See The
Thalidomide page for a further example.